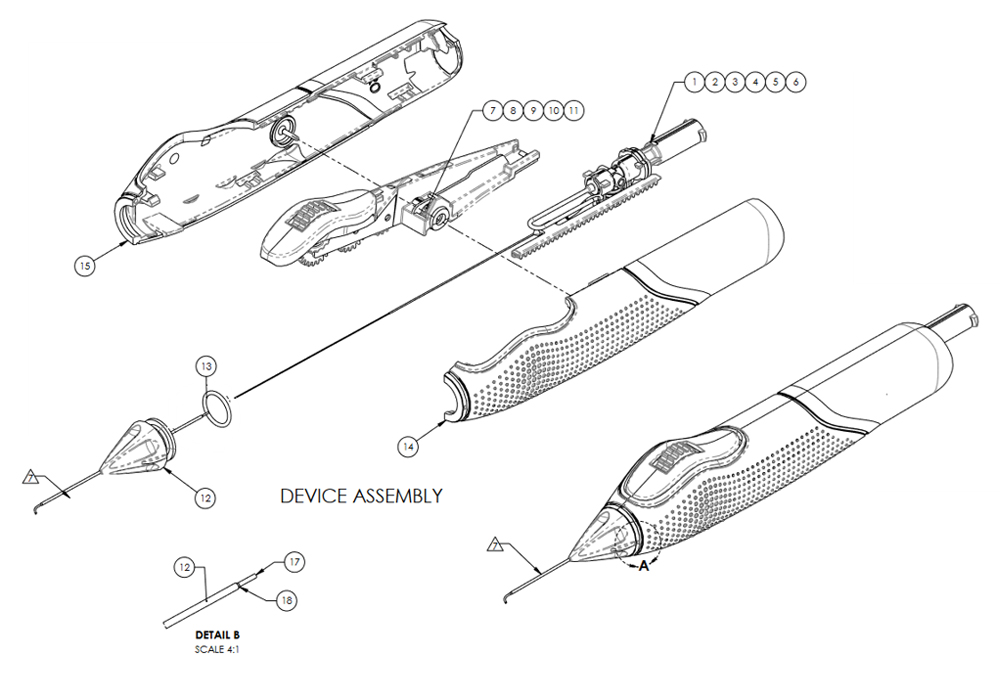
INTENDED USE: The VIA360™ Surgical System is indicated for delivery of controlled amounts of viscoelastic fluid during ophthalmic surgery. It is also indicated to cut trabecular meshwork tissue during trabeculotomy procedures.
PATIENT POPULATION: ADULT
PROJECT NAME: Vanguard
DEVELOPMENT TEAM: Joey Tran, Rich Summers (Project Lead), Dustin Tobey (Tech Lead), Harold Miller (Independent Reviewer), Cameron Kennedy, Hamid Sharifinejad, Jason Mohr, Daniel Taylor, Elyse Tighe, Eric Porteous, Wyatt Deane, Nghia Ho, Marty Segari, Erick Nunez

PRODUCT SUPPORT:
EXECUTIVE TEAM: Bilal Khan, Khalid Mansour, Raymond Kong, Joey Tran, Cristina Avalos, Brandon Blanche
COMMERCIAL TEAM: Raymond Kong, Jackie Martinez-Cearley, Michael Knutz
QUALITY AND REGULATORY TEAM: Cristina Avalos, Daniel Martell, Marty Segari, Mike Miller, Nancy Rivera, Ernesto Navarro, Alexis Lopez, Hazel Huang, Michael Waldon, Jaime Baldovino, Victor Arellano, Taj Kaur-Hurrle
SUSTAINING AND PRODUCTION TEAM: Brandon Blanche, Jonathan Menendez, Pete Albano, Erik Lazo, Nghia Ho, Oswaldo Pompa, Corey Wong, Abdul Timbo, Victor Guzman
CLINICAL AFFAIRS TEAM: Heather Reynolds, Kendra Wright, Elysia Ison, Jeremy Reimann, Mini Balaram, Dr Malik Kahook (CMA)
LEGAL TEAM: David Klann
PRODUCT DEVELOPMENT DURATION: 24 MONTHS (8/2022 – 8/2024)
REGULATORY CLEARANCE RECEIVED: FDA 510(k) K243503 FEBRUARY 14, 2025
DID YOU KNOW:
- VIA360 started out with laser markings on the catheter but the actual process of installing them deteriorated the catheter material over time.
- The colors selected for the handset were changed 3 times
- Fluid pressure inside the pump reaches 180 psi!
- The catheter mandrel is small enough that a single spool contains 7 miles of the wire. Enough for 100k devices!