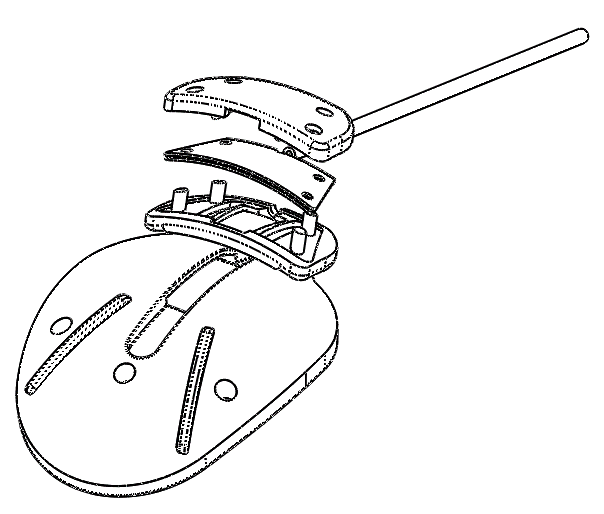
Ahmed® Glaucoma Valve and Ahmed EVO
INTENDED USE: The Ahmed® Glaucoma Valve is indicated for the management of refractory glaucomas, where previous surgical treatment has failed, or by experience is known not to provide satisfactory results. Such refractory glaucomas can include neovascular glaucoma, primary open angle glaucoma unresponsive to medication, congenital or infantile glaucoma, and refractory glaucomas
resulting from aphakia or uveitis.
PATIENT POPULATION: Adult and Pediatric
PROJECT NAME: AGV and Phoenix (EVO)
DEVELOPMENT TEAM:
- AGV: Dr. A. Mateen Ahmed
- Phoenix: Jennfer Dede (Project Lead), Patrick Chen (Project Lead), Jason Mohr (Technical Reviewer), Wyatt Dean (Tech Lead), Helena Gan, Harold Miller (Independent Reviewer), Joey Tran

PRODUCT SUPPORT:
EXECUTIVE TEAM:
- AGV: Dr. A. Mateen Ahmed
- PHOENIX: Bilal Khan, Khalid Mansour, Raymond Kong
COMMERCIAL TEAM:
- AGV: Dr. A. Mateen Ahmed, et al
- PHOENIX: Raymond Kong, Jackie Martinez-Cearly
QUALITY AND REGULATORY TEAM:
- AGV: Dr. A. Mateen Ahmed, et al
- PHOENIX: Cristina Avalos, Daniel Martell, Andrei Badea, Nancy Rivera, Taj Kaur-Hurrle
SUSTAINING AND PRODUCTION TEAM:
- AGV: Khalid Mansour, Joey Tran
- PHOENIX: Brandon Blanche, Jonathan Menendez, Pete Albano, Deric Munoz, Abdul Timbo
CLINICAL AFFAIRS TEAM:
- AGV: Dr. A. Mateen Ahmed
- PHOENIX:
LEGAL TEAM:
- AGV: Unknown
- PHOENIX: David Klann
REGULATORY CLEARANCE RECEIVED:
DID YOU KNOW:
- AGV was personally developed by Dr Ahmed when he had started an IOL company
PATENT LINKS:
- US 20170348151 A1 – Intraocular Drainage Device
- US 9381112 B1 – Bleb Drainage Device, Ophthalmological Product And Methods
- US 8632489 B1 – Implantable Medical Assembly And Methods
- US 20040215126 A1 – Device For Treating Glaucoma & Method Of Manufacture
- US 6261256 B1 – Pocket Medical Valve & Method
- US 5785674 A – Device And Method For Treating Glaucoma
- US 5681275 A – Ophthalmological Device With Adaptable Multiple Distribution Plates
- US 5071408 A – Medical Valve