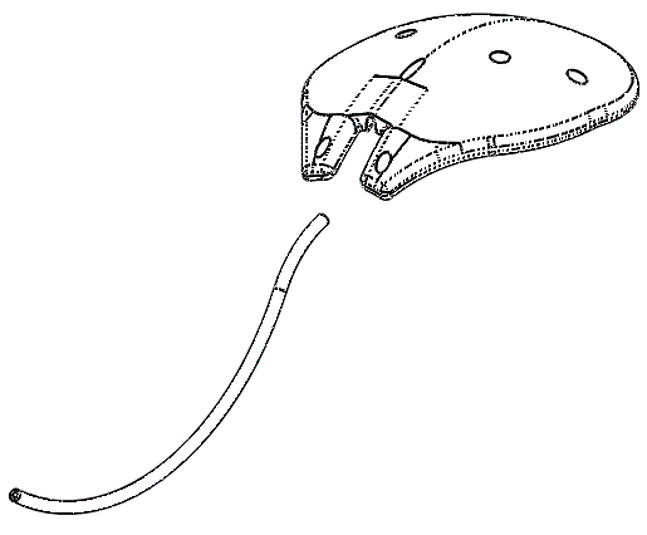
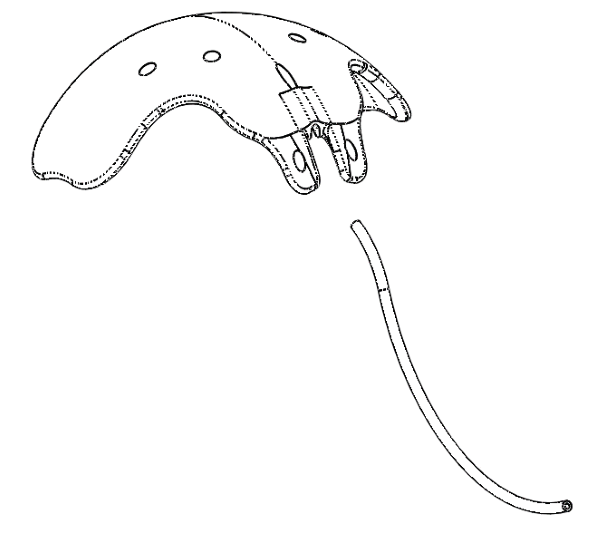
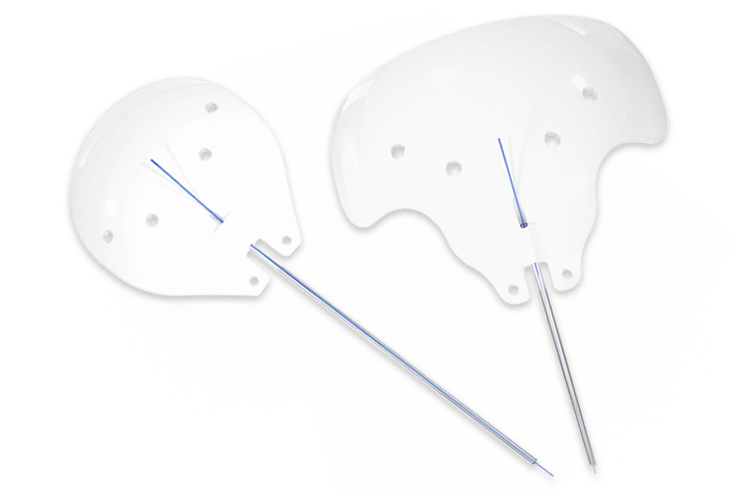
Ahmed ClearPath® and Ahmed ClearPath ST
INTENDED USE: The Ahmed ClearPath® glaucoma drainage devices are indicated for the management of refractory glaucoma where previous surgical treatment has failed or is not expected to provide satisfactory results.
PATIENT POPULATION: ADULT
PROJECT NAME: Cortez
DEVELOPMENT TEAM:
- Ahmed ClearPath: John Koontz, Joey Tran (Project and Technical Lead), Ramon Venegas Jr (Independent Reviewer), Patrick Chen, Wyatt Deane, Daniel Martell
- Ahmed ClearPath ST: Chris Kee (Project Lead), Cameron Kennedy (Co-Project Lead), Cody Jackson (Tech Lead), Jason Mohr, Harold Miller (Independent Reviewer), Helena Gan, Joey Tran
PRODUCT SUPPORT:
EXECUTIVE TEAM: Bilal Khan, Khalid Mansour, Rafael Chan, John Koontz, Cristina Avalos, Brandon Blanche, Oswaldo Pompa
COMMERCIAL TEAM: Rafael Chan, Annamarie Piccioni, Malika Singh, Ray Nix, Yasir Iqbal, Toya Ashley, Jackie Martinez-Cearley (ACP ST)
QUALITY AND REGULATORY TEAM: Cristina Avalos, Daniel Martell, Ramon Venegas, Lorena Diaz, Erick Nunez, Matt Emperado, Michael Waldon, Mukesh Sabarad
SUSTAINING AND PRODUCTION TEAM: Brandon Blanche, Oswaldo Pompa, Jonathan Menendez, Pete Albano, Erik Lazo (ACP ST)
CLINICAL AFFAIRS TEAM: Dr Malik Kahook (CMA)
LEGAL TEAM: David Klann
PRODUCT DEVELOPMENT DURATION:
- 43 MONTHS (03/2017 – 10/2020) (ACP)
- 6 MONTHS (09/2023 – 03/2024) (ACP ST)
REGULATORY CLEARANCE RECEIVED: FDA 510(k) K182518 JANUARY 18, 2019